
Visit BW® at Aquatech Amsterdam for Expert Chemical Dosing Solutions
Team Blue-White® is at Aquatech Amsterdam and ready to discuss the Best


The pH level of water plays a significant role in every stage of a municipal water treatment system, from disinfection to filtration to corrosion control. Proper pH management is essential for optimizing the performance of treatment processes. Maintaining optimal pH levels is crucial in drinking water treatment to ensure the safety and quality of the water supply.
Traditionally, liquid acids such as sulfuric acid and muriatic acid have been employed to lower pH levels. However, these chemicals pose a health and safety risk. Their caustic and flammable properties require operators to wear personal protective equipment (PPE) while handling them, and they must be carefully stored in proper conditions. As such, many water treatment plants (WTPs) are increasingly turning to carbon dioxide (CO2) dosing to manage pH levels. CO2 provides several advantages over liquid acids. In addition to being easier to use, it is also more environmentally friendly and can have long-term cost benefits.
Liquid acids use a dosing pump connected to a source container and a 4-20mA signal, the latter of which helps control how much acid is administered to the system. Hooking up and swapping out acid containers is theoretically simple but requires operators to follow explicit safety procedures and wear the proper PPE. In addition, liquid acids require additional time for complete mixing, increasing the treatment duration, which could potentially lead to an overdosing situation.
By contrast, CO2 is stored in pressurized tanks with a regulator and diffuser. When the pH rises above a set point, a solenoid valve opens to allow more CO2 to enter and lower the pH. CO2 dissolves almost instantly and distributes evenly throughout the water, resulting in faster, more efficient and precise pH control.

Liquid acids tend to lower the pH abruptly, leading to potential instability and corrosive conditions in water distribution systems that can damage pipes, fittings, and other infrastructure components and potentially introducing harmful contaminants to the water.
CO2 dosing provides a more controlled and gradual pH reduction. CO2 is selfbuffering, forming carbonic acid (H2CO3) when dissolved in water. This carbonic acid can dissociate into hydrogen ions (H+) and bicarbonate ions (HCO3-). The reversible equilibrium reaction between carbonic acid and bicarbonate ions helps maintain the pH of the solution, usually keeping it just above 6. Thus, it is difficult to over-dose the water. This reduces the risk of corrosion, minimizing the risk of water quality deterioration due to the release of metals or other pipe-related contaminants.
Of course, this also means that CO2 may not be an ideal solution for treating highly alkaline water. However, for the majority of water systems it does represent an advantage.
Another advantage of using CO2 for pH reduction is its superior environmental profile. Liquid acids pose potential risks to human health and the environment. The storage, handling, and transport of concentrated liquid acids require careful precautions to prevent accidents or spills that can result in soil and water contamination or injury to personnel.
In contrast, CO2 is a naturally occurring gas found in the atmosphere, and its use does not introduce additional chemicals or hazardous substances into the water supply. CO2 gas can be sourced from various industrial processes, such as power plants or fermentation, offering an opportunity for sustainable utilization of a byproduct.
CO2 also reduces the potential environmental impact associated with liquid acids. The self-buffering property ensures that CO2- dosed water will cause minimal harm to plant and animal life in the event of a water main break or a system flush.
CO2 dosing offers cost advantages over liquid acids in multiple ways. While the equipment has a higher up-front price tag, CO2 is naturally occurring and abundant, making it cheaper to produce than most acids. In addition, CO2 dosing is relatively simple and the tanks can be safely stored. This reduces costs associated with safety and other training, as well as PPE investment. Furthermore, the reduced corrosion potential associated with CO2 dosing extends the lifespan of distribution system components, saving on maintenance and replacement expenses in the long run.
While liquid acids like sulfuric acid and muriatic acid have been used for pH reduction for years, the associated health and safety risks are making CO2 dosing increasingly desirable. In addition, the use of CO2 better aligns with many WTP’s sustainability goals, while also offering numerous potential long-term cost benefits.
Written by:
Blue-White® Industries
714-893-8529

| Flow Ranges | |
|---|---|
| Flow Meter | |
| F-440 Polysulfone Flow Meter | |
| Injector | |
| Fine Air Diffusion | |
| Solenoid Valve | |
| Stainless Steel |

Team Blue-White® is at Aquatech Amsterdam and ready to discuss the Best

BW®’s Rich Hopkins just completed startup of this wall mount skid, equipped with Blue-White®’s
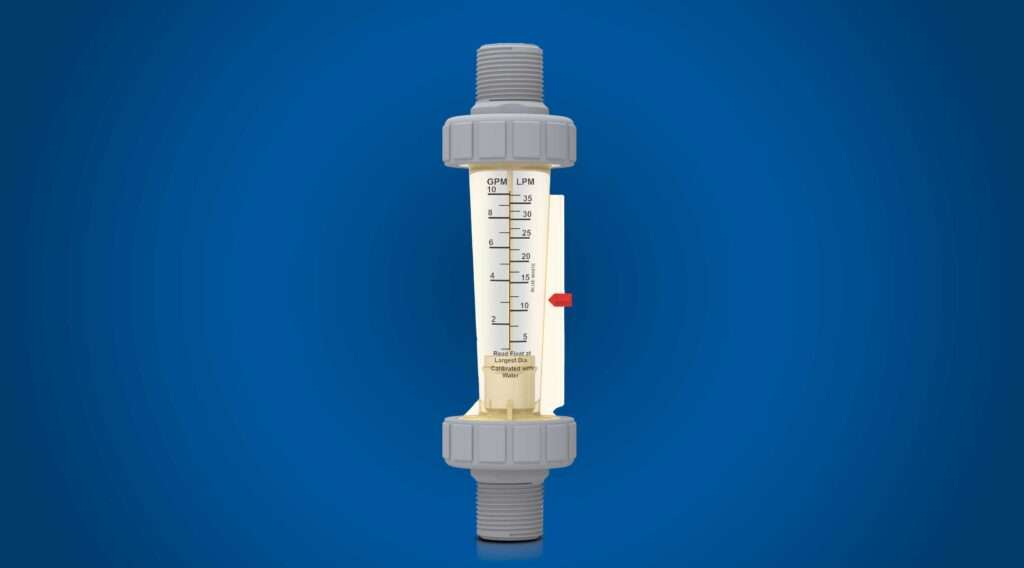
There are many industrial and municipal applications where accurately measuring fluid flow

It was a busy few days in Honolulu for the Pacific Water
Copyright © 2024 Blue-White
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Advertisement" category . |
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| CookieLawInfoConsent | 1 year | Records the default button state of the corresponding category & the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | This cookie is used by the website. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 2 years | The _ga cookie, installed by Google Analytics, calculates visitor, session and campaign data and also keeps track of site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognize unique visitors. |
| _gat_gtag_UA_85334924_1 | 1 minute | Set by Google to distinguish users. |
| _gid | 1 day | Installed by Google Analytics, _gid cookie stores information on how visitors use a website, while also creating an analytics report of the website's performance. Some of the data that are collected include the number of visitors, their source, and the pages they visit anonymously. |
| CONSENT | 2 years | YouTube sets this cookie via embedded youtube-videos and registers anonymous statistical data. |
| Cookie | Duration | Description |
|---|---|---|
| VISITOR_INFO1_LIVE | 5 months 27 days | A cookie set by YouTube to measure bandwidth that determines whether the user gets the new or old player interface. |
| YSC | session | YSC cookie is set by Youtube and is used to track the views of embedded videos on Youtube pages. |
| yt-remote-connected-devices | never | YouTube sets this cookie to store the video preferences of the user using embedded YouTube video. |
| yt-remote-device-id | never | YouTube sets this cookie to store the video preferences of the user using embedded YouTube video. |
Please fill out the form to request a quote.
A sales rep will reach out to you.
| Image | Catalog Number | Description | Price | Buy |
|---|