
Visit BW® at Aquatech Amsterdam for Expert Chemical Dosing Solutions
Team Blue-White® is at Aquatech Amsterdam and ready to discuss the Best

While chlorine level is often considered the most important measurement in commercial swimming pools, as well as water features such as waterparks, the water’s pH is just as critical. In addition to ensuring swimmer comfort, proper pH levels are key to mitigating cloudy water and scale formation, and in protecting pumps and other equipment from corrosion.
The most important role pH plays is to maximize chlorine efficiency. When the pH level drops too low (acidic), chlorine becomes more active and can dissipate quickly. If the pH is too high (alkaline), chlorine becomes less effective, allowing bacteria, algae, and other pathogens to thrive.
For years, commercial aquatics systems have relied on liquid acids to adjust pH, particularly muriatic acid, also known as hydrochloric acid. Liquid acids are highly effective pH adjusters and, when paired with the right chemical feed pumps, can aid in ensuring pH stability.
However, an increasing number of aquatics systems managers are turning to carbon dioxide (CO2) dosing for pH control. CO2 has a number of benefits that make it an appealing alternative – or, in some cases, an addition for pH balancing.
The greatest appeal of CO2 for pH reduction is its high chemical stability. When CO2 enters water, it becomes carbonic acid. Carbonic acid can further dissociate into hydrogen ions and bicarbonate ions, the former of which lowers the pH. When the pH drops below a certain point (about 6), the chemical reaction reverses to reform carbonic acid. This helps maintain the pool’s pH. More importantly, it means that equipment operators cannot accidentally over-dose the water.
Safety. CO2 is less hazardous for maintenance personnel to handle than acids, which often require workers to use protective equipment. If released into the air, CO2 is chemically neutral, making it generally safe to work with and reducing the risk of accidents and exposure to dangerous chemicals and their fumes.
Facilities with seasonal worker turnover may benefit from the reduced training and safety protocol requirements of CO2.

Reduced Corrosion. CO2 feeder systems typically result in less corrosion of pool equipment and infrastructure when compared to acids.
Minimal Impact on Total Alkalinity. Carbonic acid lowers the pH without significantly affecting total alkalinity. Total alkalinity acts as a buffer, helping to stabilize the pH level over time.
Ease of Use and Maintenance. Most CO2 feeders are simple to install, operate, and maintain. They are typically comprised of a control box, valve, flow meter, and injection fitting. Unlike acids, which are comprised of salts, CO2 will not cause buildup on the injection fitting, resulting in less maintenance over time.
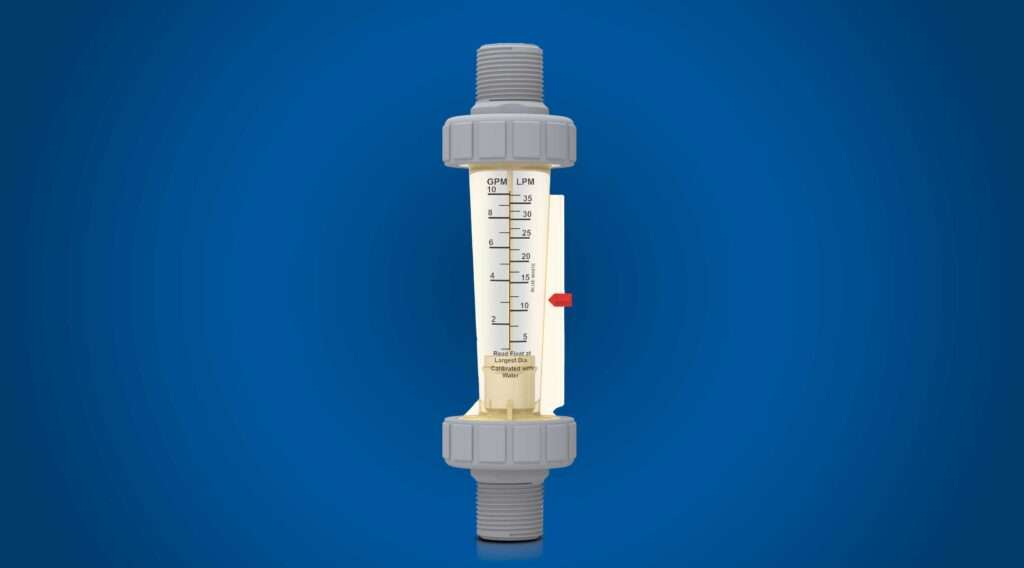
Efficiency and Precision. Although there is no harm in overdosing a pool with carbonic acid, there is no need or desire to waste it. As such, CO2 feeders can be automated and finely controlled to maintain a specific pH level. Advanced systems, like those offered by Blue-White Industries (Figure 1), come with a built-in flow meter that measures the exact amount of gas being dosed and can be used to help calibrate and validate dosing levels to reduce excess operating expenses.
Environmental Considerations. CO2 is a naturally occurring compound and is not harmful to the environment. The use of CO2 can align with sustainability goals and reduce the impact on local ecosystems.
Ease of Storage and Handling. CO2 is typically stored in cylinders as a compressed gas. These cylinders are easy to handle and safe to store. Moreover, its chemical stability means CO2 has an effectively unlimited shelf life, ensuring that product is never wasted due to expiration or improper storage.

While CO2 boasts a number of advantages over liquid acid, the decision to switch from using liquid acid depends on a range of factors. For example, for large outdoor pools, substantial rainfall can cause pH levels to rise dramatically. In such cases, CO2 may not act fast enough, and a large dose of muriatic acid may be a more effective solution. Availability can be a factor as well. Many aquatics distributors do not yet carry CO2 cannisters; instead, they may need to be obtained from beverage manufacturing suppliers.
Conversely, some states have regulations prohibiting the transportation of chlorine and acid on the same truck. This can limit supplies or increase the number of shipments needed to restock.
In many instances, it may be beneficial for an aquatics system to use both CO2 and acid for pH reduction and control. This offers the best of both worlds, allowing for efficient and stable pH control and the ability to apply a rapid correction when needed.
If the facility manager is unsure which to choose, it may be helpful to work with vendors that offer both types of systems. Such vendors can provide technical advice and support to ensure the most effective and economical solution.
Should the facility manager prefer the use of liquid acid, or choose to use both a CO2 feeder and a chemical feed pump, Blue-White’s FLEXFLO A1A chemical metering pump provides accurate and dependable performance, and requires very little maintenance.
Written by:
Blue-White® Industries
714-893-8529

Team Blue-White® is at Aquatech Amsterdam and ready to discuss the Best

BW®’s Rich Hopkins just completed startup of this wall mount skid, equipped with Blue-White®’s
There are many industrial and municipal applications where accurately measuring fluid flow

It was a busy few days in Honolulu for the Pacific Water
Copyright © 2024 Blue-White
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Advertisement" category . |
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| CookieLawInfoConsent | 1 year | Records the default button state of the corresponding category & the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | This cookie is used by the website. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 2 years | The _ga cookie, installed by Google Analytics, calculates visitor, session and campaign data and also keeps track of site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognize unique visitors. |
| _gat_gtag_UA_85334924_1 | 1 minute | Set by Google to distinguish users. |
| _gid | 1 day | Installed by Google Analytics, _gid cookie stores information on how visitors use a website, while also creating an analytics report of the website's performance. Some of the data that are collected include the number of visitors, their source, and the pages they visit anonymously. |
| CONSENT | 2 years | YouTube sets this cookie via embedded youtube-videos and registers anonymous statistical data. |
| Cookie | Duration | Description |
|---|---|---|
| VISITOR_INFO1_LIVE | 5 months 27 days | A cookie set by YouTube to measure bandwidth that determines whether the user gets the new or old player interface. |
| YSC | session | YSC cookie is set by Youtube and is used to track the views of embedded videos on Youtube pages. |
| yt-remote-connected-devices | never | YouTube sets this cookie to store the video preferences of the user using embedded YouTube video. |
| yt-remote-device-id | never | YouTube sets this cookie to store the video preferences of the user using embedded YouTube video. |
Please fill out the form to request a quote.
A sales rep will reach out to you.
| Image | Catalog Number | Description | Price | Buy |
|---|